Carthamin Proposed Biosynthesis on:
[Wikipedia]
[Google]
[Amazon]
Carthamin is a natural red pigment derived from safflower ('' Carthamus tinctorius''), earlier known as carthamine.De Candolle, Alphonse. (1885.
''Origin of cultivated plants.''
D. Appleton & Co.: New York, p. 164. Retrieved on 2007-09-25. It is used as a dye and a food coloring. As a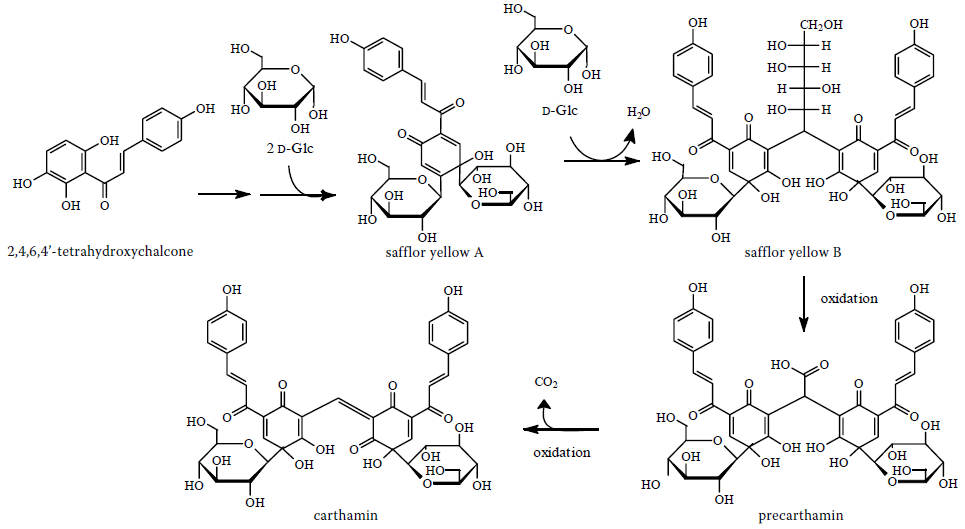
''Origin of cultivated plants.''
D. Appleton & Co.: New York, p. 164. Retrieved on 2007-09-25. It is used as a dye and a food coloring. As a
food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salt ...
, it is known as Natural Red 26.
Safflower has been cultivated since ancient times, and carthamin was used as a dye in ancient Egypt. It was used extensively in the past for dyeing wool for the carpet industry in European countries, and in the dyeing of silk and the creation of cosmetics in Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
, where the color is called ; however, due to the expensive nature of the dye, Japanese safflower dyestuffs were sometimes diluted with other dyes, such as turmeric and sappan. It competed with the early synthetic dye fuchsine as a silk dye after fuchsine's 1859 discovery.
Carthamin is composed of two chalconoid
Chalconoids Greek: χαλκός ''khalkós'', "copper", due to its color), also known as ''chalcones'', are natural phenols related to chalcone. They form the central core for a variety of important biological compounds.
They show antibacterial, ...
s; the conjugated bonds being the cause of the red color. It is derived from precarthamin by a decarboxylase. It should not be confused with carthamidin, another flavonoid.
The carthamin is biosynthesized from a chalcone
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids.
Chemical properties
Chalcones have two absorption maxima a ...
( 2,4,6,4'-tetrahydroxychalcone) and two glucose molecules to give safflor yellow A and with other glucose molecule, safflor yellow B. The next step is the formation of precarthamin and finally carthamin.

References